Sirnaomics Initiates Phase Ⅱb Study Using STP705 for Treatment of Squamous Cell Skin Cancer
Company Expects to Report Initial Clinical Data in Second Half of 2021

Sirnaomics, Inc., a biopharmaceutical company engaged in the discovery and development of RNAi therapeutics against cancer and fibrotic diseases, today announced the initiation of the Phase 2b study of the company’s lead drug candidate, STP705, for the treatment of squamous cell skin cancer.
The randomized, double-blind, placebo-controlled study will evaluate the safety and efficacy of intralesional injection of STP705 in 100 adult patients with squamous cell carcinoma in situ (isSCC). This is a two-part dose escalation trial; in the run-in period, the 30 ug and 60 ug dosing regimens from the Phase 2a study of STP705 will be further evaluated, in addition to a third new dose level. The second part of the trial will further evaluate the two most efficacious dosing regimens. The primary endpoint of this trial is the proportion of participants with histological clearance of treated isSCC lesion at the end of treatment. Histological clearance will be defined as the absence of detectable evidence of isSCC tumor cell nests as determined by central pathology review.
“In the recently concluded Phase 2a clinical trial of STP705 in isSCC, a high rate of patients achieved histological clearance in a dose dependent manner, which is the gold standard for skin cancer,” said Patrick Lu, Ph.D., the founder, President and CEO of Sirnaomics. “As we initiate the Phase 2b trial, we are hopeful to learn more about the potential of this non-surgical, non-invasive treatment for common non-melanoma skin cancer, and more broadly the promise of RNAi therapeutics in oncology.”
“isSCC continues to be a disease with high unmet therapeutic need where surgery is still considered the only viable treatment option for many patients,” said Michael Molyneaux M.D., Chief Medical Officer of Sirnaomics. “We hope to build on the success we have seen in the Phase 2a study, where we achieved 90% histological clearance rates in the 30 ug and 60 ug dosing groups. We anticipate having an interim data readout late second half of 2021 that will guide our clinical development for this indication.”
Additional information about this clinical trial is available at clinicaltrials.gov using the identifier: NCT04844983.
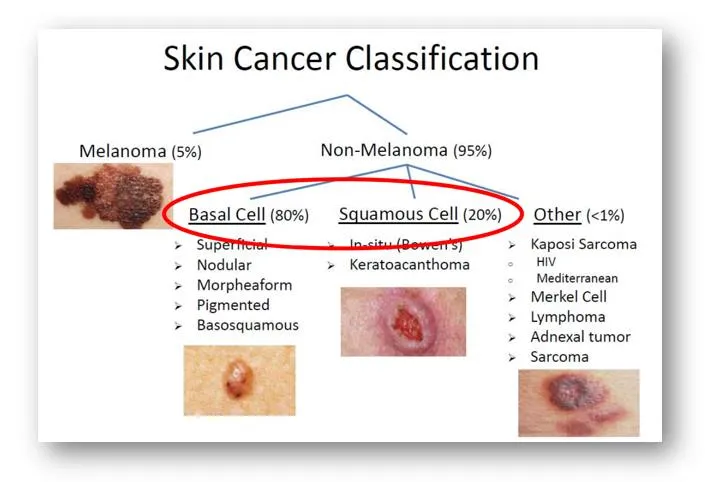
About Non-melanoma Skin Cancer and Squamous Cell Carcinoma In Situ
Skin cancer is the most common type of all cancers diagnosed each year in the United States. It is estimated that nearly half of cancers diagnosed every year will be skin cancers. Over the past decade, the incidence of skin cancers has increased dramatically. According to the JAMA Dermatology paper (Rogers, et. al. JAMA Dermatol. 2015151(10):1081-1086), an estimated 3.3 million people in the US suffer from non-melanoma skin cancer (NMSC) along with 5.43 million people that are currently living with cancer lesions. Data on specific types of NMSC were 2.55 million cases for basal cell carcinoma (47%): 2.57 million cases for squamous cell carcinoma including squamous cell carcinoma in situ (46.7%), plus another 332,000 cases of unspecified type of skin cancers.
A World Health Organization authorized report from “International Agency for Research on Cancer” (2019) has demonstrated that the number of deaths in 2018 globally for both men and women from NMSC is 65,155, where the mortality of Asia NMSC patients represents 41.9% of the global total, significantly more than other individual areas.
Squamous cell carcinoma in situ, also called Bowen disease, is the earliest form of squamous cell skin cancer (SCC). Along with basal cell carcinoma, SCC is one of two major subtypes of NMSC. The key driver for development of SCC is ultraviolet rays from the sun. It is believed that development of SCC is linked closely to genomic perturbations, genetic mutations, and altered expression of key molecules (e.g., overexpression of TGF-β1 and COX-2) that impacts squamous cell lineage commitment and terminal differentiation.
Surgery is the currently the most common treatment option for the treatment of NMSC. The various forms of surgical modalities carry significant cutaneous adverse events, risk of scar, infection and bleeding. Surgery can also have a significant recurrence rate. As a result, there is a high unmet need for an FDA approved local injection therapy that is safe and effective.

About STP705
Sirnaomics’ leading product candidate, STP705, is a siRNA (small interfering RNA) therapeutic that takes advantage of a dual-targeted inhibitory property and polypeptide nanoparticle (PNP)-enhanced delivery to directly knock down both TGF-β1 and COX-2 gene expression. The product candidate has received multiple IND approvals from both the US FDA and Chinese NMPA, including treatments of cholangiocarcinoma, nonmelanoma skin cancer and hypertrophic scar. STP705 has also received Orphan Drug Designation for treatment of cholangiocarcinoma and primary sclerosing cholangitis. STP705 is currently in four clinical trials for different indications. A Phase 2a study of STP705 for treatment of squamous cell skin cancer (isSCC) in adult patients demonstrated positive efficacy and safety results, with 76% of all patients (19/25) achieving complete histologically clearance and the two optimal dosing ranges achieving 90% histological clearance of tumor cell in the lesion. No significant or serious adverse events, including no significant cutaneous skin reactions, were reported in the study, and the company was able to define a clear therapeutic window in advance of later stage studies.
Read the original news release
Sirnaomics, Inc., a leading privately held biopharmaceutical company for discovery and development of RNAi therapeutics, is a Delaware corporation headquartered in Gaithersburg, Maryland, USA, with subsidiaries in Suzhou and Guangzhou, China. The Company’s mission is to develop novel therapeutics to alleviate human suffering and advance patient care in areas of high unmet medical need. The guiding principles of the company are: Innovation, Global Vision with a Patient Centered focus. Members of the senior management team have a great deal of combined experience in the biopharmaceutical industry, financial, clinical and business management in both the USA and China. The company is supported by funding from institutional investors and corporate partnerships. Sirnaomics has developed a strong portfolio of intellectual property with an enriched product pipeline. The therapeutic areas of focus include oncology and anti-fibrotic therapeutics. Learn more at www.sirnaomics.com.





 Back to Press Release
Back to Press Release
 Previous
Previous
