Who We Are
Sirnaomics is the first and only clinical-stage RNA therapeutics biopharmaceutical company with a strong presence in both China and the U.S. that is discovering and developing innovative drugs for indications with significant unmet medical needs and large market opportunities. We have built a professional international team for the discovery and development of RNAi therapeutics and mRNA vaccines and therapeutics, based on our proprietary drug delivery technology platforms.
Our subsidiary RNAimmune develops mRNA-based vaccines and therapeutics.







Leadership Team












Our Story

9issued patents in China

9issued patents in the U.S.

2issued patents in Europe

119pending patent applications, including:
19 Chinese patent applications
43 U.S. patent applications
(including 32 U.S. provisional patent applications)
8 patent applications under the Patent Cooperation Treaty
6 patent applications in Europe
43 patent applications in other jurisdictions.
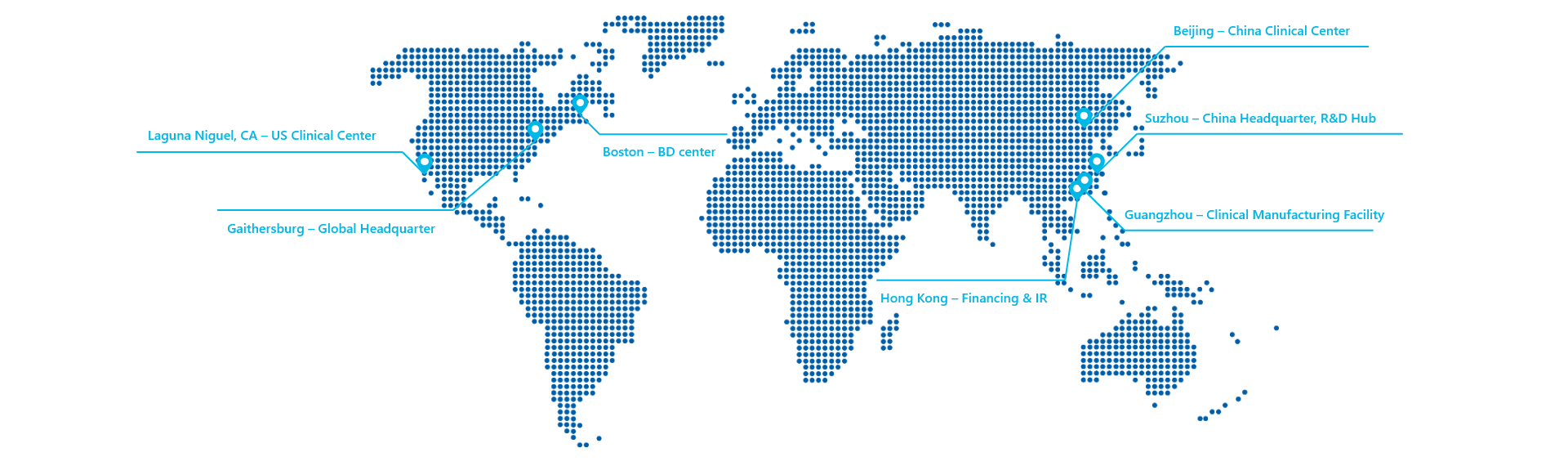
401 Professional Dr, STE 280 Gaithersburg, MD 20879, USA
218 Xinghu Street, STE A4-415 BioBay, Suzhou, China
12 Luoxuan 3 Road, STE 4-306 Guangzhou International Bio-island Guangzhou, China
Sirnaomics Ltd.
Sirnaomics (Hong Kong) Limited
46/F, Hopewell Centre, 183 Queen's Road East, Wanchai, Hong Kong, China
Sirnaomics, Boston
245 First Street, 18F
Cambridge, MA 02142, USA




