The first and only clinical-stage DNA-based COVID-19 vaccine candidate in China. pGX9501 has potentially favorable immunogenicity, a better safety and tolerability profile, an advanced manufacturing process and strong stability, differentiating it from other COVID-19 vaccines approved and in development. We commenced phase II clinical trial in December 2020 and conducted a phase III clinical trial for pGX9501 in the second half of 2021 in the worldwide with our strategic partner.

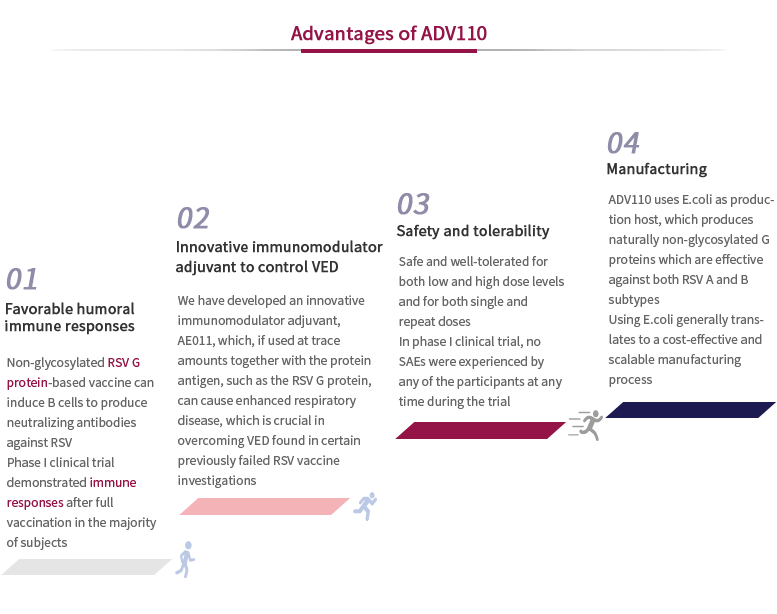
A potential first-in-class protein subunit RSV vaccine candidate with a novel adjuvant, designed to protect children aged 6 months to 5 years old and the elderly over 65 years old. To date, our ADV110 is the only clinical-stage RSV vaccine candidate designed and developed by Chinese companies and one of the most clinically-advanced globally. We are also the first in clinical stage to use a novel adjuvant that acts as an immunomodulator in RSV vaccines, potentially expanding the use of adjuvants beyond its traditional role of enhancing immunogenic response in vaccines. We have commenced the preparation of subject enrollment for our phase II clinical trial and the first participants have been successfully dosed in Australia.
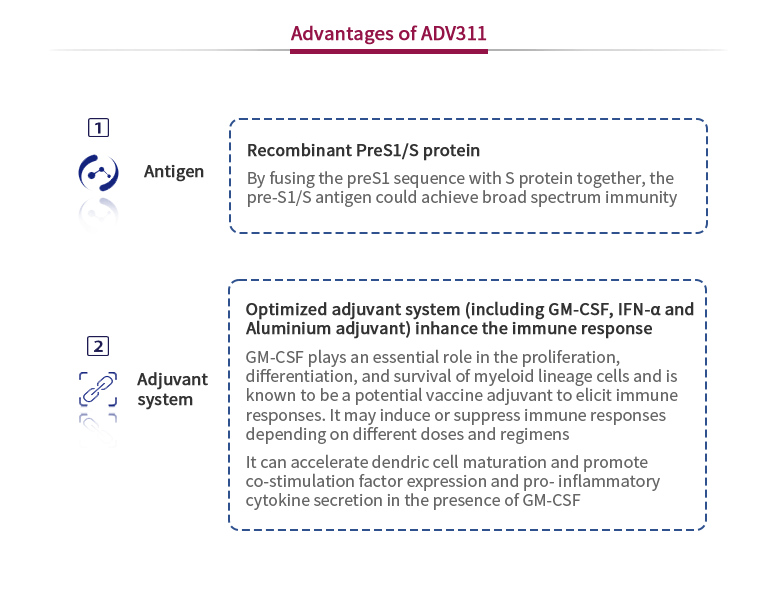
Hepatitis B is a widespread and infectious liver disease caused by HBV. Most adults infected with HBV can self-heal but children infected with HBV could easily develop chronic hepatitis, which could later lead to cirrhosis, liver failure and liver cell carcinoma. Patients with HBV present with a high level of disease burden, including social stigma, labor loss, and heavy economic burden. ADV311 is a preclinical-stage therapeutic HBV vaccine that is a potential functional cure for CHB patients. A preclinical-stage therapeutic vaccine that is a potential functional cure. In investigator-initiated clinical trials of an MOA-equivalent version of our ADV311, the HBsAg (hepatitis B surface antigen, which is a surface antigen of the HBV) negative conversion rate, a strong indicator of a functional cure for HBV, in our vaccine treatment group was 15.4%, which is higher than other treatment groups in CHB patients. Other clinical-stage HBV therapeutic vaccines have not recorded such high functional cure statistics. We expect to submit investigational new drug (IND) application to the NMPA in 2022.